Introduction:
Letermovir is a terminase complex inhibitor that was recently approved for prevention of cytomegalovirus (CMV) reactivation after allogeneic hematopoietic cell transplant (HCT). Its favorable side effect profile makes it an attractive alternative to other anti-CMV agents. Nevertheless, its use may present other challenges secondary to enzyme induction, leading to clinically significant drug-drug interactions. Voriconazole is widely used for prophylaxis against invasive fungal infections in the setting of HCT. By virtue of its hepatic metabolism mediated by CYP2C19, the package insert recommends close monitoring of voriconazole trough concentration when given concomitantly with letermovir. The objective of this study was to characterize the extent of interaction between voriconazole and letermovir in allogeneic HCT recipients. The study was approved by the institutional review board.
Methods:
Patient selection and data collection: An institutional database was queried to identify patients who underwent allogeneic HCT and received antifungal prophylaxis with voriconazole from November 2018 through July 2020. Per the institutional standard operating procedure (SOP), patients initially received intravenous micafungin 50 mg daily which was switched to voriconazole when oral drug administration became feasible. The voriconazole prophylactic dose was guided by CYP2C19 phenotype. Rapid metabolizers (harboring the gain of function variantCYP2C19*17) received 300 mg twice daily whereas others received 200 mg twice daily. Further dose adjustment was warranted if trough concentration, measured at least 5 days after starting voriconazole, did not fall within target range of 1 to 5.5 mg/L. Patients at risk for CMV reactivation who underwent HCT after SOP revision (November 2019) received prophylaxis with letermovir 480 mg daily on day +6 post HCT.
After a retrospective review of electronic medical records, HCT recipients were divided into two cohorts;cohort 1included patients who received letermovir prophylaxis whereascohort 2comprised those who did not. Data extracted from medical records included: demographics, hematological disorder, voriconazole dose and trough concentration, and date of letermovir initiation.
Statistical analysis: The Student's t-test was used to compare mean voriconazole trough plasma concentration between cohorts 1 and 2. The chi-squared/Fisher's exact test was used to compare rate of subtherapeutic voriconazole trough concentration. Multivariate logistic regression analysis was performed to determine the association between letermovir use and subtherapeutic voriconazole concentration after adjusting for age, gender, race, weight and voriconazole dose. All statistical analyses were performed in SAS (version 9.4)
Results:
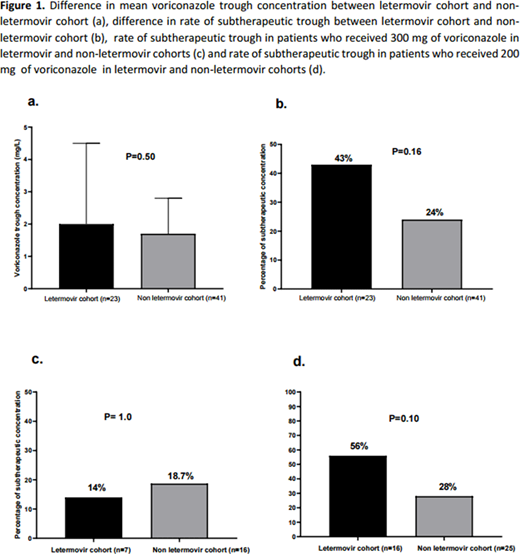
64 patients were identified (23 in cohort 1 and 41 in cohort 2). Baseline characteristics were comparable except for age (62.0±8.7 years in cohort 1 vs. 58.0±12.2 years, p=0.01); 39% of patients in cohort 1 and 30% in cohort 2 received voriconazole 300 mg twice daily upfront for prophylaxis due to the rapid CYP2C19 metabolizer phenotype whereas the rest received voriconazole 200 mg twice daily (p=0.6). There was no significant difference in mean voriconazole trough plasma concentration (p=0.5) or frequency of subtherapeutic trough (p=0.16) between cohorts 1 and 2 (Figure 1). Multivariate logistic regression analysis indicated that letermovir prophylaxis had no impact on subtherapeutic voriconazole concentration (OR: 0.4, 95% CI: 0.1-1.4, p=0.1). In the subgroup of patients who received voriconazole at 300 mg twice daily, the rate of subtherapeutic concentration did not differ significantly between cohorts 1 and 2 (p=1.0), whereas a non-significant trend in rate of subtherapeutic voriconazole concentration was noted in subgroup of patients who received the 200 mg dose (p=0.1,Figure 2)
Conclusions:
Our single center experience suggests there may not be a significant interaction between voriconazole and letermovir. Notably, patients receiving the 300 mg dose upfront may not require an additional dose increase to achieve a voriconazole trough within the recommended range despite the concomitant use of letermovir. Our group is collaborating with other centers to corroborate these findings, particularly in patients receiving the standard voriconazole prophylactic dose of 200 mg.
Grunwald:Forma Therapeutics:Research Funding;Agios:Consultancy;Abbvie:Consultancy;Trovagene:Consultancy;Daiichi Sankyo:Consultancy;Astellas:Consultancy;Daiichi Sankyo:Consultancy;Trovagene:Consultancy;Trovagene:Consultancy;Premier:Consultancy;Astellas:Consultancy;Astellas:Consultancy;Genentech/Roche:Research Funding;Premier:Consultancy;Genentech/Roche:Research Funding;Genentech/Roche:Research Funding;Premier:Consultancy;Janssen:Research Funding;Merck:Consultancy;Forma Therapeutics:Research Funding;Incyte:Consultancy, Research Funding;Celgene:Consultancy;Incyte:Consultancy, Research Funding;Incyte:Consultancy, Research Funding;Pfizer:Consultancy;Celgene:Consultancy;Celgene:Consultancy;Cardinal Health:Consultancy;Merck:Research Funding;Daiichi Sankyo:Consultancy;Agios:Consultancy;Abbvie:Consultancy;Merck:Consultancy;Amgen:Consultancy;Merck:Consultancy;Amgen:Consultancy;Abbvie:Consultancy;Pfizer:Consultancy;Amgen:Consultancy;Pfizer:Consultancy;Agios:Consultancy;Cardinal Health:Consultancy;Forma Therapeutics:Research Funding;Cardinal Health:Consultancy;Janssen:Research Funding.Ai:Incyte:Speakers Bureau;Celgene:Speakers Bureau.Knight:Foundation for Financial Planning:Research Funding.Chojecki:Incyte:Research Funding;Novartis:Other: Investigator Meeting Attendance.Avalos:Juno:Membership on an entity's Board of Directors or advisory committees;Best Practice-Br Med J:Patents & Royalties: receives royalties from a coauthored article on evaluation of neutropenia.Copelan:Amgen:Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal